Phet isotopes and atomic mass answer key – Embark on an enlightening journey into the realm of isotopes and atomic mass, guided by the comprehensive Phet simulation and answer key. This dynamic exploration delves into the fundamental concepts of isotopic variation, atomic mass calculation, and their profound applications in chemistry and beyond.
Through interactive simulations and expert analysis, we unravel the intricacies of isotopes, their impact on atomic mass, and the practical significance of these concepts in fields ranging from medicine to nuclear science. Join us as we decipher the secrets of isotopic abundance, mass spectrometry, and the fascinating world of isotopes in nature and technology.
Isotopes and Atomic Mass: Definition and Concepts
Isotopes are variations of an element that have the same number of protons but differ in the number of neutrons. This difference in neutron count affects the mass of the atom, leading to different isotopes of the same element. Atomic mass is the weighted average mass of all isotopes of an element, considering their relative abundances.
Calculating Atomic Mass, Phet isotopes and atomic mass answer key
Atomic mass is calculated by multiplying the mass of each isotope by its abundance and summing the products. The formula for atomic mass is:
Atomic mass = (Mass of isotope 1 × Abundance of isotope 1) + (Mass of isotope 2 × Abundance of isotope 2) + …
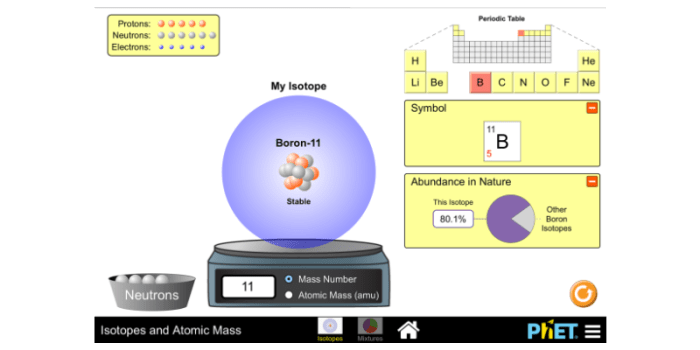
Phet Simulation: Overview and Functionality
The Phet simulation “Isotopes and Atomic Mass” is an interactive tool that allows users to explore the concepts of isotopes and atomic mass. Key features include:
- Visual representation of isotopes with varying neutron counts
- Interactive sliders to adjust isotopic abundances
- Calculation of atomic mass based on user-defined abundances
Answer Key Analysis: Understanding the Simulation
The answer key for the Phet simulation tests key concepts such as:
- Identifying isotopes based on neutron count
- Calculating atomic mass from isotopic masses and abundances
- Understanding the relationship between atomic mass and isotopic composition
Application in Chemistry: Isotopic Abundance and Mass Spectrometry
Isotopes and atomic mass have practical applications in chemistry, including:
- Determining isotopic abundance using mass spectrometry
- Understanding the behavior of elements in chemical reactions
- Tracing the movement of elements in environmental and biological systems
Real-World Examples: Isotopes in Nature and Technology
Isotopes are used in various fields, such as:
- Medicine: Radioisotopes for medical imaging and treatment
- Geology: Isotopic ratios for dating rocks and understanding geological processes
- Nuclear science: Isotope separation for nuclear power and weapons
FAQ Resource: Phet Isotopes And Atomic Mass Answer Key
What is the significance of isotopic ratios in understanding natural processes?
Isotopic ratios serve as valuable indicators of past environmental conditions, geological events, and biological processes. By analyzing the relative abundance of different isotopes in natural samples, scientists can reconstruct ancient climates, trace the movement of tectonic plates, and uncover the evolutionary history of species.
How is mass spectrometry employed to determine isotopic abundance?
Mass spectrometry is a powerful analytical technique that separates ions based on their mass-to-charge ratio. By measuring the relative intensities of different ion peaks, scientists can determine the isotopic composition of a sample with high precision and accuracy. This information is crucial for various applications, including environmental monitoring, forensic science, and medical diagnostics.