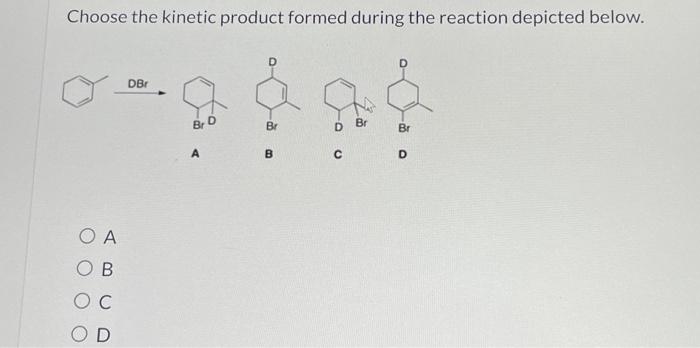
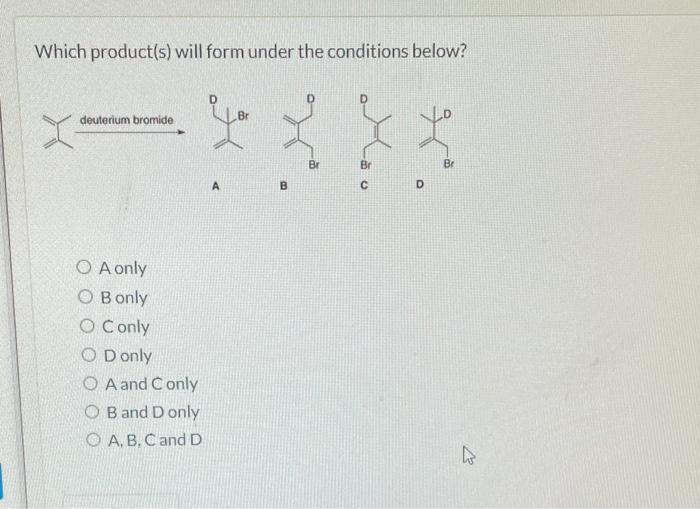
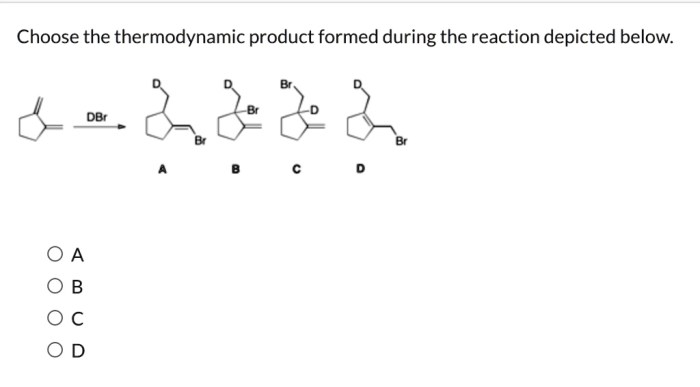
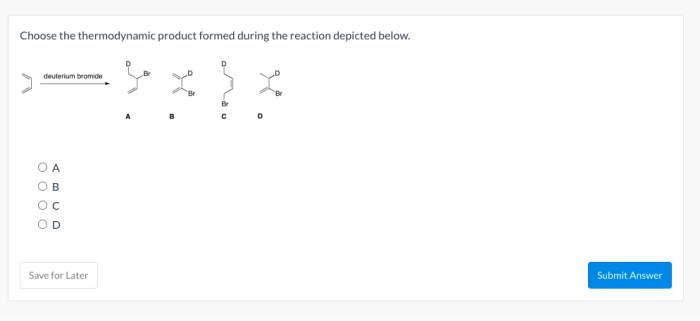
Choose the thermodynamic product formed during the reaction depicted below. This question delves into the fascinating realm of thermodynamics, where we explore the intricate relationship between energy and chemical reactions. Thermodynamics provides a powerful framework for understanding and predicting the behavior of chemical systems, enabling us to harness their potential for technological advancements and scientific discoveries.
By delving into the concepts of Gibbs free energy, reaction equilibrium, and the factors that influence product formation, we gain invaluable insights into the driving forces behind chemical reactions. This knowledge empowers us to predict the outcomes of reactions, design experiments, and optimize industrial processes, ultimately shaping the future of chemistry and its applications.
Introduction to Thermodynamics: Choose The Thermodynamic Product Formed During The Reaction Depicted Below.
Thermodynamics is the study of energy and its transformation in chemical reactions. It plays a vital role in predicting the direction and outcome of chemical reactions.
The first law of thermodynamics states that the total energy of an isolated system remains constant. Enthalpy (H) represents the total thermal energy of a system, while entropy (S) measures the degree of disorder or randomness.
Gibbs Free Energy and Reaction Equilibrium
Gibbs free energy (G) is a thermodynamic potential that combines enthalpy and entropy. It is defined as G = H – TS.
A reaction is at equilibrium when the Gibbs free energy change (ΔG) is zero. A negative ΔG indicates a spontaneous reaction, while a positive ΔG indicates a nonspontaneous reaction.
Factors Affecting Product Formation
Temperature, pressure, and concentration influence reaction equilibrium.
- Temperature: Increasing temperature favors endothermic reactions (ΔH > 0) and disfavors exothermic reactions (ΔH< 0).
- Pressure: Increasing pressure favors reactions that result in a decrease in the number of gas molecules.
- Concentration: Increasing the concentration of reactants favors the forward reaction, while increasing the concentration of products favors the reverse reaction.
Methods for Predicting Product Formation
Predicting the thermodynamic product formed in a reaction can be done using several methods:
- Equilibrium constants: Equilibrium constants relate the concentrations of reactants and products at equilibrium and can be used to predict the extent of reaction.
- Reaction spontaneity: A negative ΔG indicates a spontaneous reaction, and the product will be the one with the lowest free energy.
- Hess’s law and bond enthalpies: By manipulating bond enthalpies, one can estimate the enthalpy change of a reaction and predict the product.
FAQ Section
What is the significance of Gibbs free energy in predicting product formation?
Gibbs free energy is a thermodynamic potential that combines enthalpy and entropy, providing a measure of the spontaneity of a reaction. A negative Gibbs free energy indicates a spontaneous reaction, favoring the formation of the thermodynamic product.
How does temperature influence product formation?
Temperature affects the equilibrium constant of a reaction, which in turn determines the relative amounts of reactants and products. Generally, increasing temperature shifts the equilibrium towards products with higher entropy.
What is the role of Hess’s law in predicting product formation?
Hess’s law states that the enthalpy change of a reaction is independent of the pathway taken. This allows us to calculate the enthalpy change of a reaction by summing the enthalpy changes of individual steps, providing a convenient method for predicting product formation.